As I have done several times during the pandemic, I am again forwarding the report our Medical Director, Marjorie Newman, MD, sends out weekly to keep our medical staff informed about COVID-19. It is a bit more technical than some other reports we have sent to patients, but it does such a good job of capturing what is happening now that I am attaching it in basically the same form we use internally.
You'll read some statistical data about cases nationwide, in California and in Santa Barbara County. Dr. Newman also discusses important vaccine and booster updates for Pfizer, Moderna and Johnson & Johnson. You will also find information about how we are providing flu vaccines and COVID vaccines and boosters at Sansum Clinic.
While we have made a lot of progress in managing COVID-19 in our County, further progress ultimately hinges on more individuals getting vaccinated and everyone continuing to observe safety precautions including mask wearing and social distancing. We've come a long way since the first of these letters, but it is not yet over and continuing to be careful will allow us all to get closer to a full return to normal.
Thank you for choosing Sansum Clinic for your care, and remember, we are still smiling under our masks.
CEO and CMO
From: Marjorie Newman, MD
Sent: Tuesday, October 19, 2021 8:38 AM
Subject: Weekly COVID-19 Update

October 19, 2021
To: Colleagues
From: Marjorie Newman, MD
Re: Weekly COVID-19 Update –More about Boosters
Overview: The good news is that reports of new cases continue to trend steadily downward, with approximately 86,000 new cases being identified each day in the past week, down from more than 160,000 daily cases at the beginning of September. The number of individuals hospitalized due to COVID-19 is also on the decline with 63,200 (down from 68,000 last week) and the number of deaths per day is down ~16% at 1600 per day. While much of the country is rebounding from the summer Delta surge, Colorado, Vermont and Michigan are among a handful of states seeing sustained case growth and Alaska continues to lead the country in number of cases per capita (125 new cases per 100,000).
In the US, the pace of vaccination has remained steady with ~843,000 vaccine doses administered per day last week-including the first, second and additional or booster doses. Approximately 68% of U.S. adults (age 18 and older) are fully vaccinated and 84% of those 65 and older are fully vaccinated. 67% of those age 12 and older are fully vaccinated. However, only 57% of all Americans are fully vaccinated and 67% of all who are eligible to receive vaccine (12 and older) are fully vaccinated.
Below is a graph from the Johns Hopkins Coronavirus Resource Center depicting the trend in cases in the ten most impacted countries. The U.S. (green trend line) demonstrates a decline in cases post summer Delta surge, with new daily cases hovering around 100,000 per day in the past 14 days. The U.S. still has the highest number of new daily cases compared to the other 9 most impacted countries.
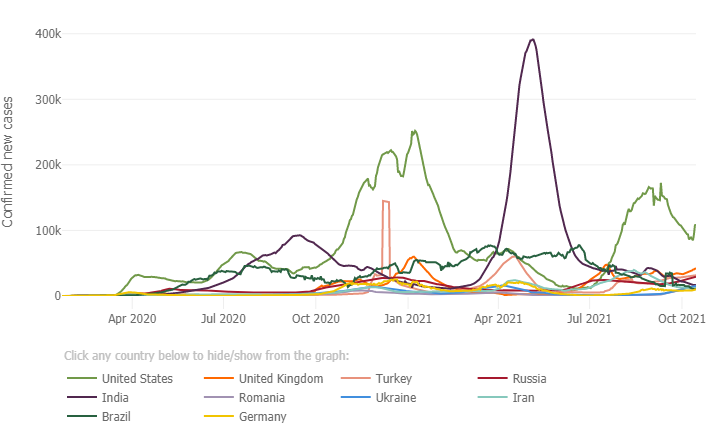
California Update:
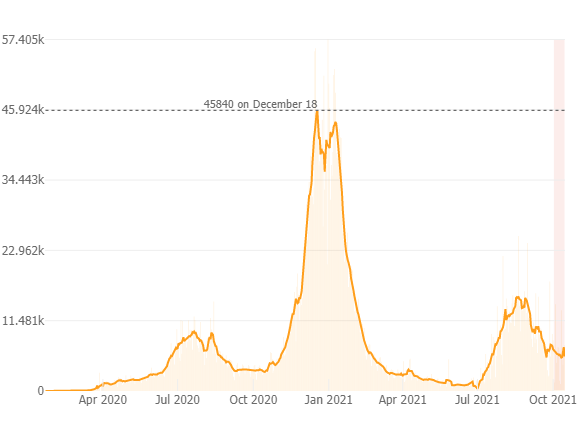
As of October 16th since the pandemic began there has been ~4.57 million positive COVID-19 cases reported in California; 70,150 deaths and 51 million vaccines administered. Below is a graph from the Johns Hopkins COVID-19 Resource webpage depicting the 7 day average number of new cases and the light pink bar indicates a slight increase in cases over the past week, but overall a downward trend in new cases, now with ~5000 new cases per day (11.7 new cases per 100K), and a test positivity rate of 2.3 % with 4,200 people hospitalized throughout the state (down 25% from 14 days ago), and 100 new deaths which is a 6% increase over the past 14 days. In CA 60.2% of the population is fully vaccinated and 72% of the eligible population is vaccinated. As we know, vaccines prevent serious illness, save lives and reduce further spread of COVID-19. As more people are vaccinated the virus is less likely to spread, mutate and potentially become even more dangerous.
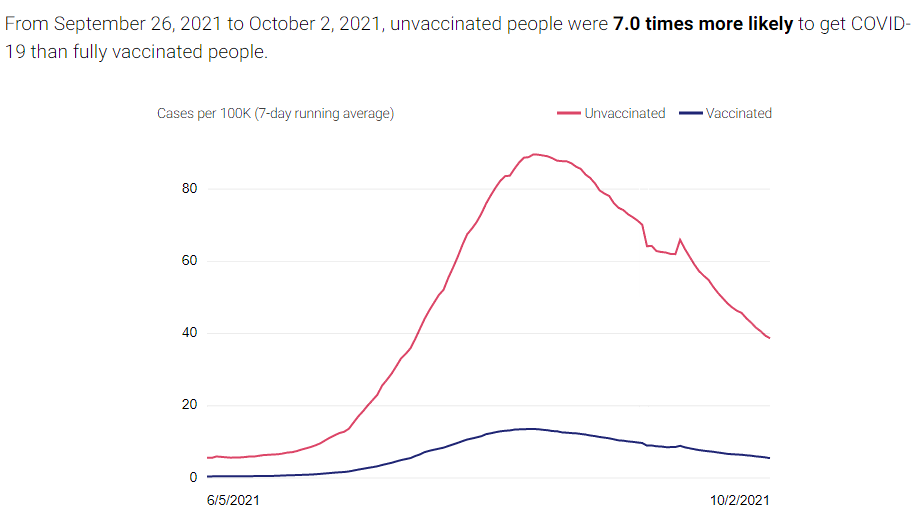
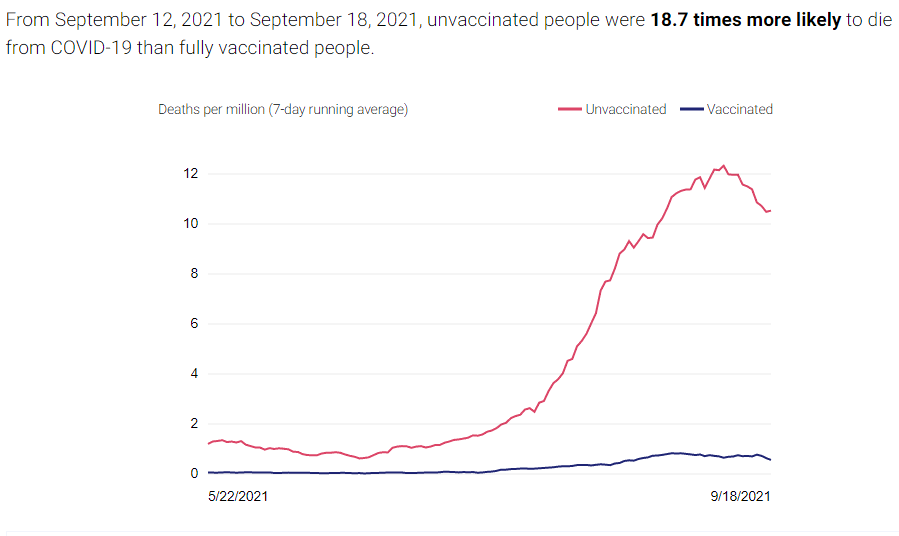
There are two compelling graphs below from the California Department of Public Health (CDPH), which compare the unvaccinated to the vaccinated population in CA and the rates of new cases and deaths between the two. A vaccinated individual is a person who received two doses of the Pfizer or Moderna vaccines or one dose of the Janssen vaccine at least 2 weeks before they tested positive for COVID-19. The graphs below use data from people age 16 and older collected over the last 120 days.
Santa Barbara County:
According to the Santa Barbra County Public Health Department website, as of Tuesday October 19th there are currently 262 active infections with 39 new cases reported. The test positivity rate in SB county is 2.6% (down from 3.1%). There are now 35 patients in the hospital with 13 in the ICU and seven more deaths since the last update, for the cumulative total of 516 deaths in Santa Barbara County due to COVID-19 since the onset of the pandemic.
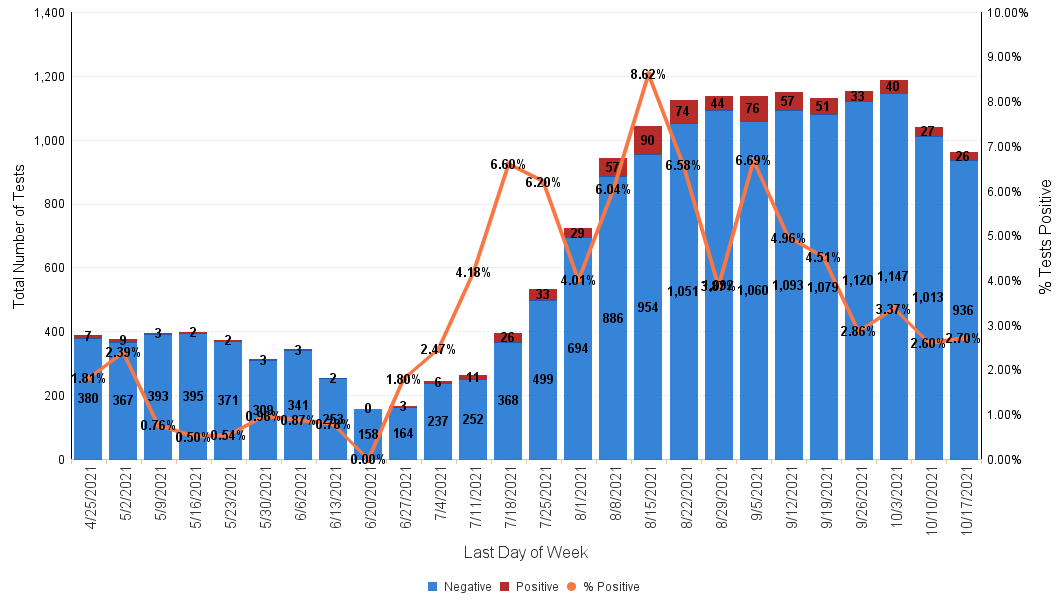
Below is the Sansum Clinic weekly COVID-19 testing data for the week ending October 17th. Our percent positive has hovered around the 2%-3% range in the past three weeks, now holding steady at 2.70%.
COVID-19 Vaccinations in Santa Barbara County: As of October 18th 562,519 vaccines have been administered, with 59.3% of the county now fully vaccinated and 70% of those eligible to receive vaccine (age 12 and up) are now fully vaccinated and 77.9 % of those eligible have received at least one dose.
A mentioned last week, the Santa Barbara County Health Officer extended the prior Health Officer Order regarding face coverings indicating that face coverings are to be worn at all times in all indoor public settings and while inside any business through November 4th at which time it will be reassessed. According the Santa Barbara Public Health Department, face coverings will be required until the county is below a case rate of 6 per 100,000 cases and a test positivity of less than 2%. Currently the county is at 8.2 cases per 100,000 and a positivity rate of 2.6%.
------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
UPDATES & REMINDERS:
Pfizer BioNTech COVID-19 Vaccine Booster Doses:
The CDC recommends the following populations should receive a booster shot6 months following their second dose of their Pfizer-BioNTech COVID-19 vaccine:
• People 65 years and older;
• Residents in long-term care settings; and
• People aged 50–64 years with underlying medical conditions
The CDC recommends the following populations may receive a booster shot 6 months following their second dose of their Pfizer-BioNTech COVID-19 vaccine:
• People aged 18-49 years with underlying medical conditions; and
• People aged 18-64 years who are at increased risk for COVID-19 exposure and transmission because of occupational or institutional setting (health care worker, front line workers, teachers, etc.).
Persons that meet the CDC guidelines above are able to access their booster does in locations that are providing vaccine.
Johnson &Johnson COVID-19 Vaccine Booster Doses:
The FDA’s independent advisers met last week (October 14th and October 15th) to discuss greenlighting booster doses of the J&J vaccine. The FDA Vaccine Advisory Committee unanimously approved a booster dose of the Johnson &Johnson vaccine for all Americans 18 years and older who received a single dose, at least two months after the first dose, indicating that it will provide the best protection against infection. Researchers found that among more than 600,000 veterans, J&J's vaccine's protection fell from 88% in March to 3% in August. As a result, many experts have indicated that the J&J vaccine should probably have been a two dose vaccine to begin with given the more robust and lasting immune response that the second dose is providing. About 15 million Americans have received the J&J vaccine, according to the US Centers for Disease Control and Prevention, and nearly 91% of them received it more than two months ago. As the greenlight looms for another dose of Johnson & Johnson's Covid-19 vaccine, experts are urging those who received it to get a booster shot as soon as it's available because it will provide them with the best protection against the coronavirus. Although the FDA advisory committee has voted to approve the second dose, the CDC’s independent advisory committee meeting is slated for later this week, October 20th and 21st to provide finalized guidance. We will keep you updated as we learn more.
Moderna COVID-19 Vaccine Booster Doses:
The FDA’s independent advisers met last week (October 14th and October 15th) to discuss greenlighting booster doses of the Moderna vaccine. Studies indicated that a third Moderna shot at half the dosage (50 micrograms as opposed to 100 micrograms) used for the first two dose series was safe and produced a strong immune response and indicated that boosters should prevent breakthrough infections. The FDA Vaccine advisory committee unanimously recommended giving booster shots of Moderna’s Covid-19 vaccine to people ages 65 and older and other vulnerable Americans. More than 69 million people originally received the Moderna vaccine. The CDC’s independent advisory committee meeting is slated for later this week, October 20th and 21st to provide finalized guidance on who would be eligible to receive a Moderna booster. We will keep you updated as we learn more.
Mixing vaccines for booster doses:
The National Institutes of Health presented information to the FDA from an ongoing study showing that it didn't matter which vaccine people got first and which booster vaccine they received -- it was safe to “mix and match.” One study found that recipients of Johnson & Johnson’s single-dose shot who received a Moderna booster saw their antibody levels rise 76-fold in 15 days, compared with only a fourfold increase after an extra dose of Johnson & Johnson. Reports in the media indicate that the Food and Drug Administration is planning to allow individuals to receive a different Covid-19 vaccine as a booster than the one initially received and it is anticipated that this approval may happen by the end of this week or early next week. The FDA will likely not recommend one shot over another, and it is expected that they may indicate that using the same vaccine as a booster, when possible is preferable, but it is anticipated that vaccine providers will likely be able to use their discretion to offer a different formulation. We will keep you updated as this evolves.
Pfizer Vaccine for children ages 5-11- The FDA Advisory Committee will meet next week on October 26th to review data for the Pfizer vaccine for children ages 5-11, followed by the CDC/ACIP panel convening on November 2nd and 3rd to make official recommendations. Pfizer has proposed giving children 1/3 of the adult dosage. This may require adding more diluent to each injection or using a different vial or syringe. We will keep you posted as we learn more.
How are we managing influenza vaccine and COVID-19 booster doses at Sansum Clinic?
Eligible patients do not need an order from their provider for a Pfizer booster dose. Patients are able to use MyChart to self-schedule for their COVI-19 vaccine booster, if they are age 18 or older, had a prior Pfizer series more than 6 months ago and indicate that they are at high risk for severe disease based upon medical condition or occupation. We have notified patients of the ability to self- schedule through e-mail. Also, patients may call the COVID hotline number at (805) 681- 7805 to schedule a booster if they don’t utilize MyChart. In addition, we will be posting the attached COVID vaccine flyer, which is also on the website.
• We will be continuing our Friday afternoon COVID-19 vaccine clinics where we had been providing third doses to those who are immunocompromised and will now expand that to those who are 18 or older and have completed their Pfizer vaccine series more than 6 months ago and are eligible to receive a booster dose.
• In addition, we will be utilizing the 215 Pesetas Lane Immunization Tent and have expanded to offer COVID-19 Pfizer booster doses starting this week (Wednesdays and Thursdays). Patients are able to self-schedule for the influenza immunization (administered on Mondays and Tuesdays) or the Pfizer COVID-19 booster (administered on Wednesdays or Thursdays and expanding to include Tuesdays next week as additional booster formulations are likely to be approved).
• In addition to the Immunization Tent and Friday afternoon COVID vaccine clinics, we are liberalizing the distribution of the Pfizer vaccine at several of our clinic locations to allow providers to offer COVID-19 vaccine (including booster doses) to appropriate patients at already scheduled clinic appointments. We are working on staff training to ensure that they meet the CDC requirements and certifications for handling and administering the vaccine and will add the COVID-19 vaccine order set to each department that will be providing vaccine. The goal is to have staff trained at virtually all of our primary care locations in order to be able to administer vaccine to anyone who is eligible at the time of a scheduled appointment. We would recommend that you ask your patient early on in the appointment whether they want a booster (assuming they are eligible), as that will provide time for the patient to be monitored for the remaining 15 minutes after the vaccine is administered.
• Once the CDC ACIP and CDPH outline guidance regarding booster doses for Moderna and Johnson & Johnson (presumably by the end of this week) we will expand our booster efforts to include these formulations at the immunization tent (Tuesdays, Wednesdays and Thursdays), Friday afternoon COVID-19 vaccine clinic at 215 Pesetas Lane, as well as administration at all primary care office visits.
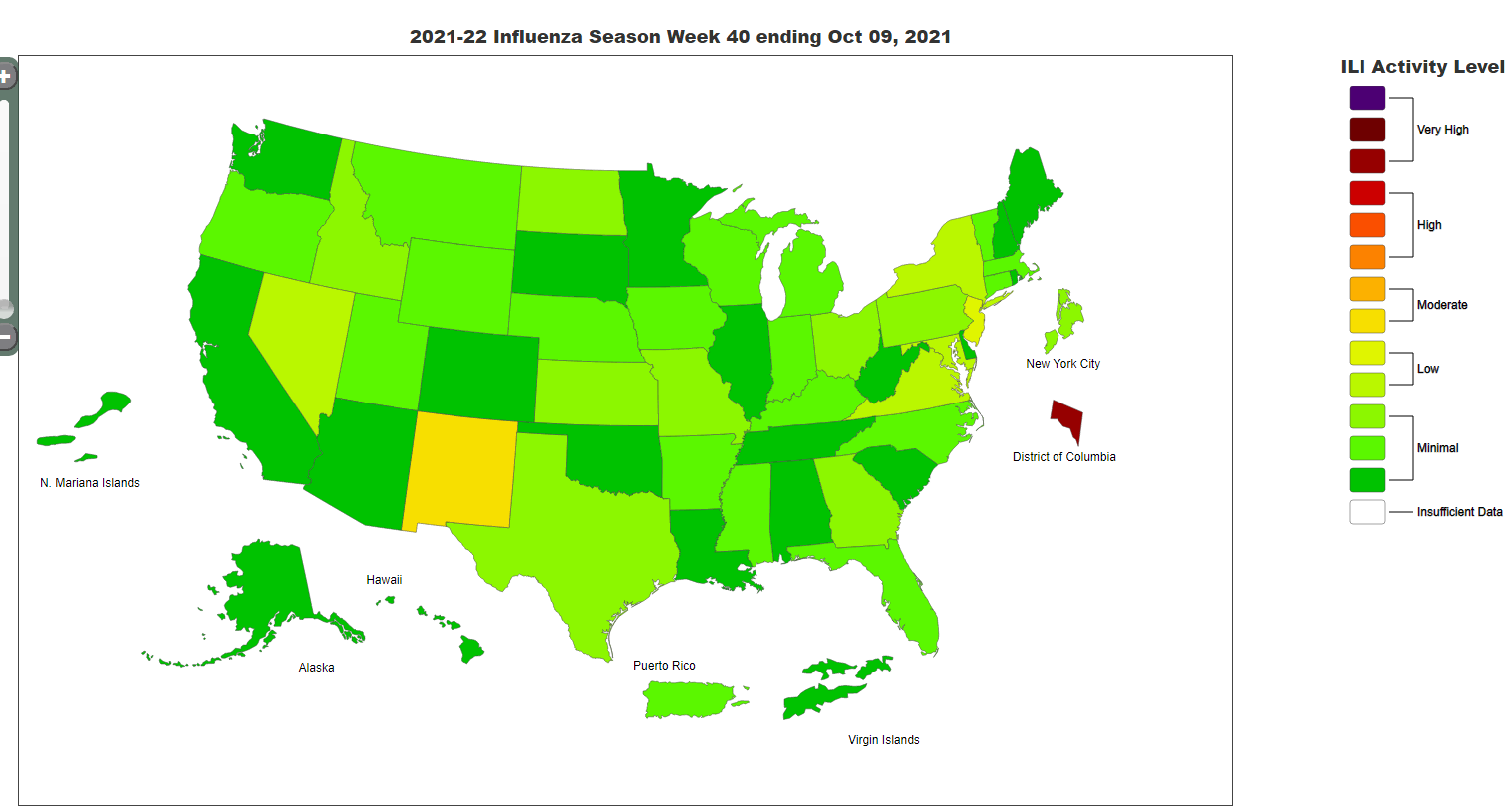
Seasonal influenza activity in the US remains low (see influenza activity map from the CDC website below). We will continue to provide influenza vaccine on Mondays at the Immunization tent, as well as at office visits in primary care departments. Please encourage all of your patients age 6 months and older to get a flu vaccine.
We hope the above information has been helpful and please don’t hesitate to contact me if you have any questions.
Marjorie Newman MD
Medical Director
Sansum Clinic